

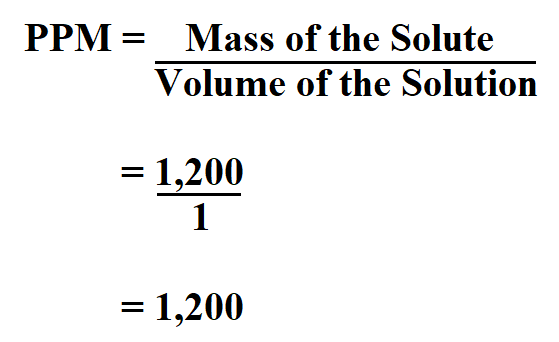
So, for the sake of explaination, let's say your "40% solution" contains 40 mls of 100% ethyl alcohol in 100 mls of water (by the way this is also called an 80 PROOF alcoholic beverage, since 'Proof' is 2 X % alcohol!). To calculate PPM, understand that % is Parts/100 (expressed in a number of ways as stated above which does not change the value of the PPM answer).

Further, your 40 % solutions could be 40 gms of an active substance blended with 60 mls of another liquid or 40gms of a substance added to 60 gms of another type of substance or 40 mls of a substance blended with 60 mls of another substance, respectively, such that the total solution in all cases makes up 100mls of the final blend. Like the previous answer-% is parts per 100 in any physical form you choose (e.g.


 0 kommentar(er)
0 kommentar(er)
